Background The Italian national program of pediatric-inspired chemotherapy GIMEMA LAL1913 trial for adult Ph- acute lymphoblastic leukemia (Ph- ALL) has reported favorable results particularly in the younger subgroup of patients (pts) aged 18-40 years (Bassan et al, Blood Adv 2023). After the closure of the trial, this treatment scheme was adopted in the clinical practice by many Italian hematology centers for the treatment of newly diagnosed adult Ph- ALL. The safety of this intensive treatment in terms of infectious complications deserves to be investigated in the real life.
Aims To evaluate in the context of the real life the incidence of infectious complications in Ph- ALL pts treated off protocol according to the GIMEMA LAL1913 chemotherapy scheme and to assess the impact of the prophylactic measures adopted.
Methods We retrospectively collected data on 178 Ph- ALL pts treated in 13 Italian hematology centers participating to the Campus ALL network in Italy. Pts were homogeneously treated following the GIMEMA LAL1913 scheme, but the use of anti-infective prophylaxis differed among centers and among cycles. We analyzed data with regard to the type and site of the infections, the cycles of treatment during which the infections occurred. Finally, we correlated the incidence of the infections with the presence or absence of anti-infective prophylaxis.
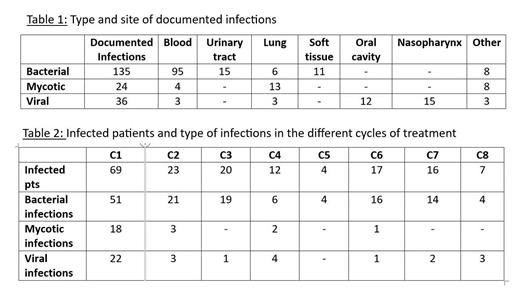
Results A total of 301 infectious events were diagnosed during the treatment of 134 of the 178 Ph- ALL pts (75%), including 98 episodes of fever of unknown origin (FUO) reported in 58 pts (33%). The distribution of the different types of documented infections for a total of 195 is reported in Table 1. The severity grading of infections (CTCAE.5) was available in 137 events and was ≥3 in 101. SARS COV2 infection accounted for 22% of the viral infections (8/36 cases). We diagnosed 24 pulmonary infections (in 21 pts): 15 mycotic (13 probable and 2 possible), 6 bacterial and 3 viral infections. The number of pts undergoing an infection/cycle is shown in Table 2: 69 patients had at least one infection during cycle 1 (39% of treated pts); infections were mainly bacterial and occurred in prevalence during cycle 1. Two patients died of sepsis by Pseudomonas aeruginosa during cycle 1 (mortality rate: 1.1%). Pts receiving antibacterial prophylaxis were 94/178 (53%). Bacterial infections were reported in 47 pts receiving prophylaxis and in 41 pts who did not undergo prophylaxis; the difference between the two groups was not significant (p: 0.8). Patients receiving antifungal prophylaxis were 119/178 (67%). Mycotic infections were reported in 14 pts, and in 10 pts who did not receive prophylaxis; again, no significant difference was recorded between the two groups (p: 0.3). Patients receiving antiviral prophylaxis were 151/178 (85%). Viral infections were reported in 30 pts and in 4 pts not receiving prophylaxis; no statistical difference was observed (p: 0.4).
Summary/Conclusion The intensive GIMEMA LAL1913 treatment applied in the real life to newly diagnosed adult Ph- ALL pts is associated with a non-negligible rate of infections; >75% of treated pts suffered from at least one infection. Moreover, the large majority of these infections were severe with a CTCAE.5 grading ≥3. However, the infection-related mortality was very low (1.%); the anti-infective treatment together with the achievement of a complete hematologic recovery were able to control and resolve the infectious complications. Bacterial infections were prevalent and bacteremias/sepsis represented 70% of events. Cycle 1 was most affected by infectious complications of all types; mycotic and viral infections were mostly reported during cycle 1, while they decreased markedly during the following cycles. Also incidence of bacterial events decreased during treatment, though they continued to occur also in the late phases of the management suggesting that a high level of attention should be maintained up to the end of treatment. Anti-infective prophylaxis was heterogeneously applied, but it remains unclear if it is necessary. The first analysis of this survey does not show an advantage of prophylaxis in reducing infections; however, particularly for fungal complications, the numbers are too limited to consider these results as conclusive. Data collection is still ongoing, and a larger sample size will allow more robust conclusions.
Disclosures
Zappasodi:Amgen, Pfizer, Abbvie, Astellas: Honoraria. Cerrano:Insight Novartis Servier Abbvie Janssen Jazz Astellas Italfarmaco: Honoraria. Fracchiolla:Abbvie, Jazz, Pfizer, Amgen: Speakers Bureau; Abbvie, Jazz, Pfizer, Amgen: Other: travel grants. Papayannidis:Abbvie, Astellas, Servier, Menarini/Stemline, BMS, Pfizer, Amgen, Janssen, Incyte, Novartis: Honoraria; Pfizer, Astellas, Janssen, GSK, Blueprint, Jazz Pharmaceuticals, Abbvie, Novartis, Delbert Laboratoires: Membership on an entity's Board of Directors or advisory committees. Chiaretti:Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Candoni:Abbvie: Honoraria; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal